top of page


Research & Development
DR background
Diabetic Retinopathy (DR)
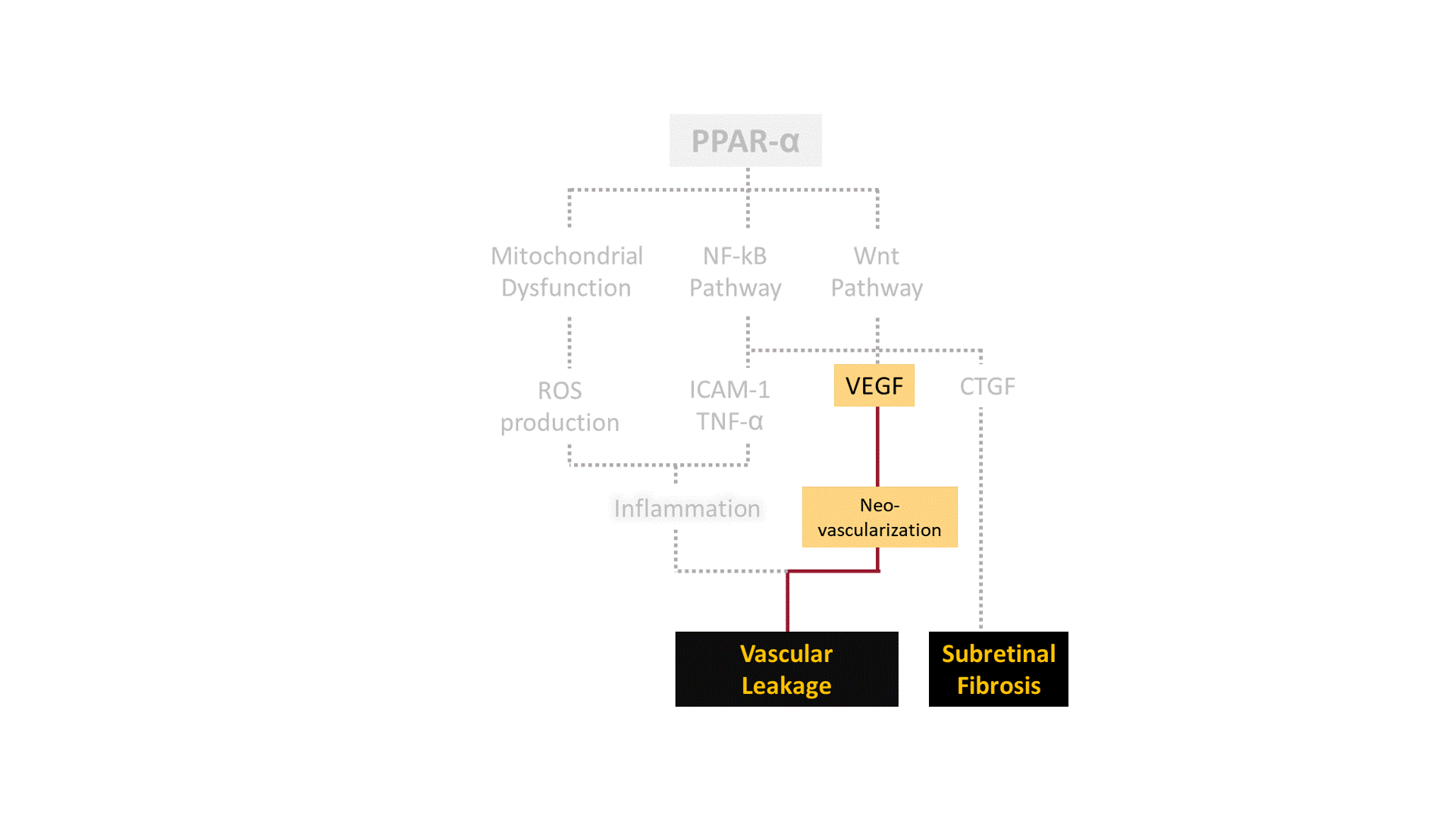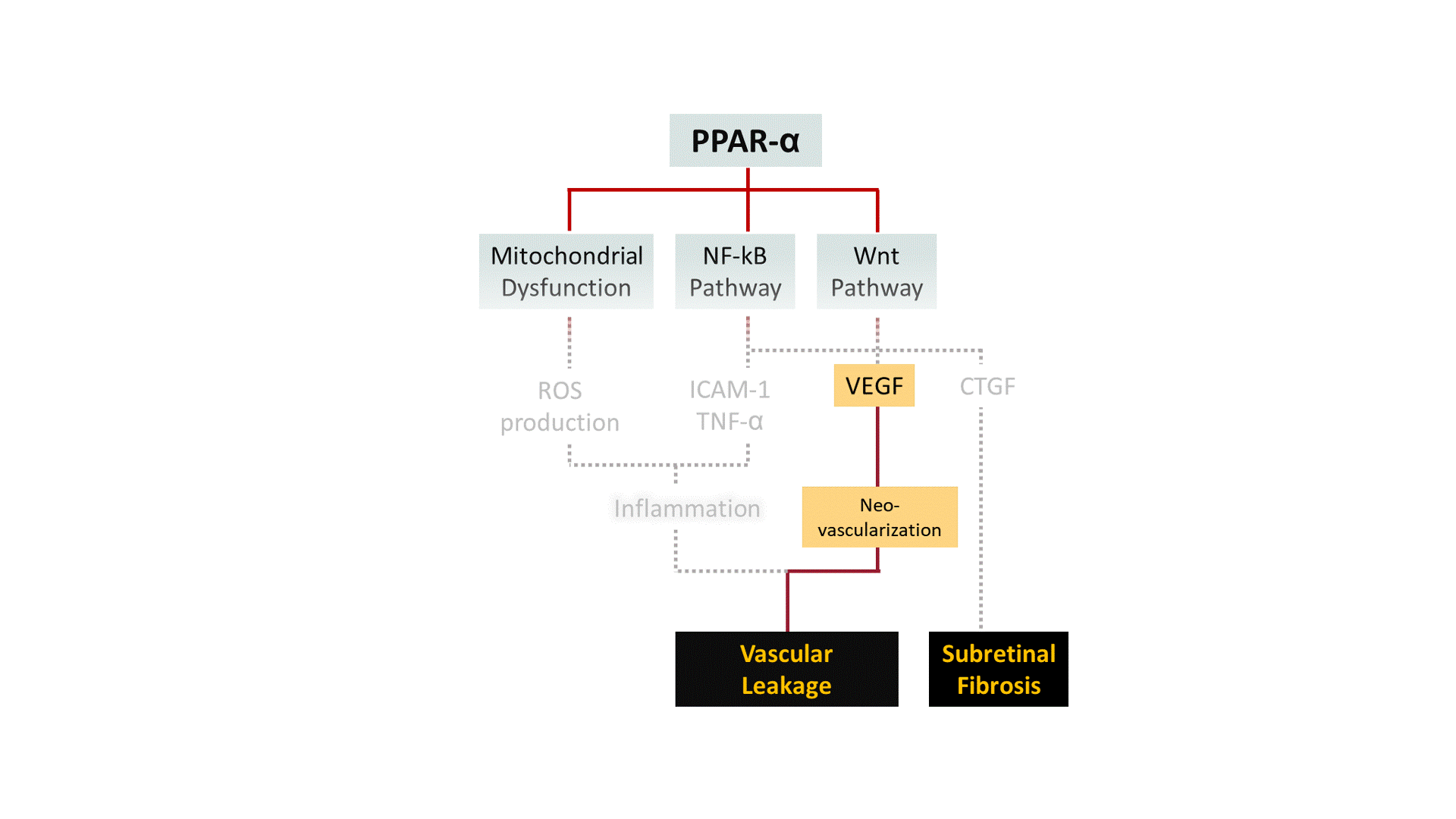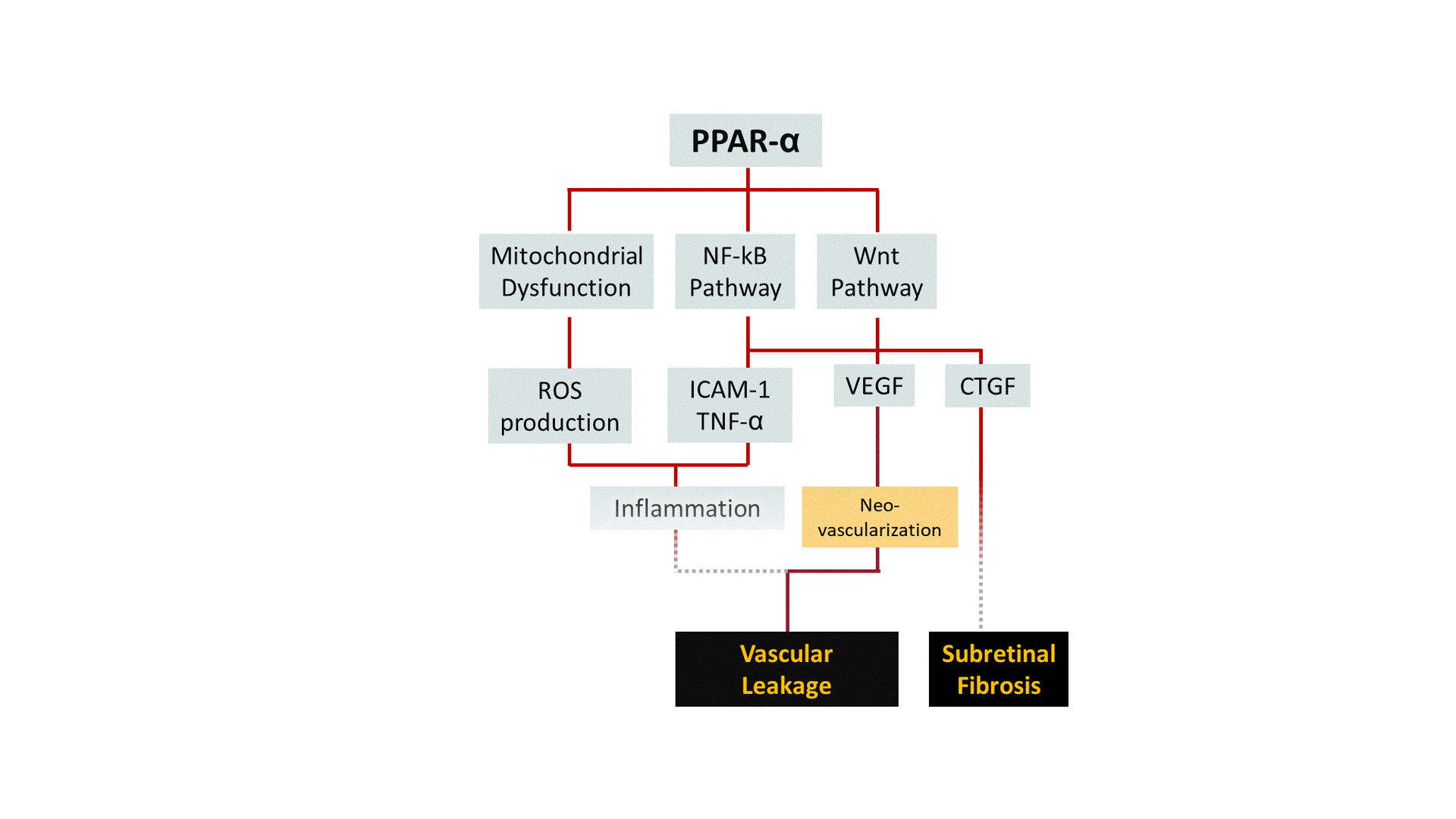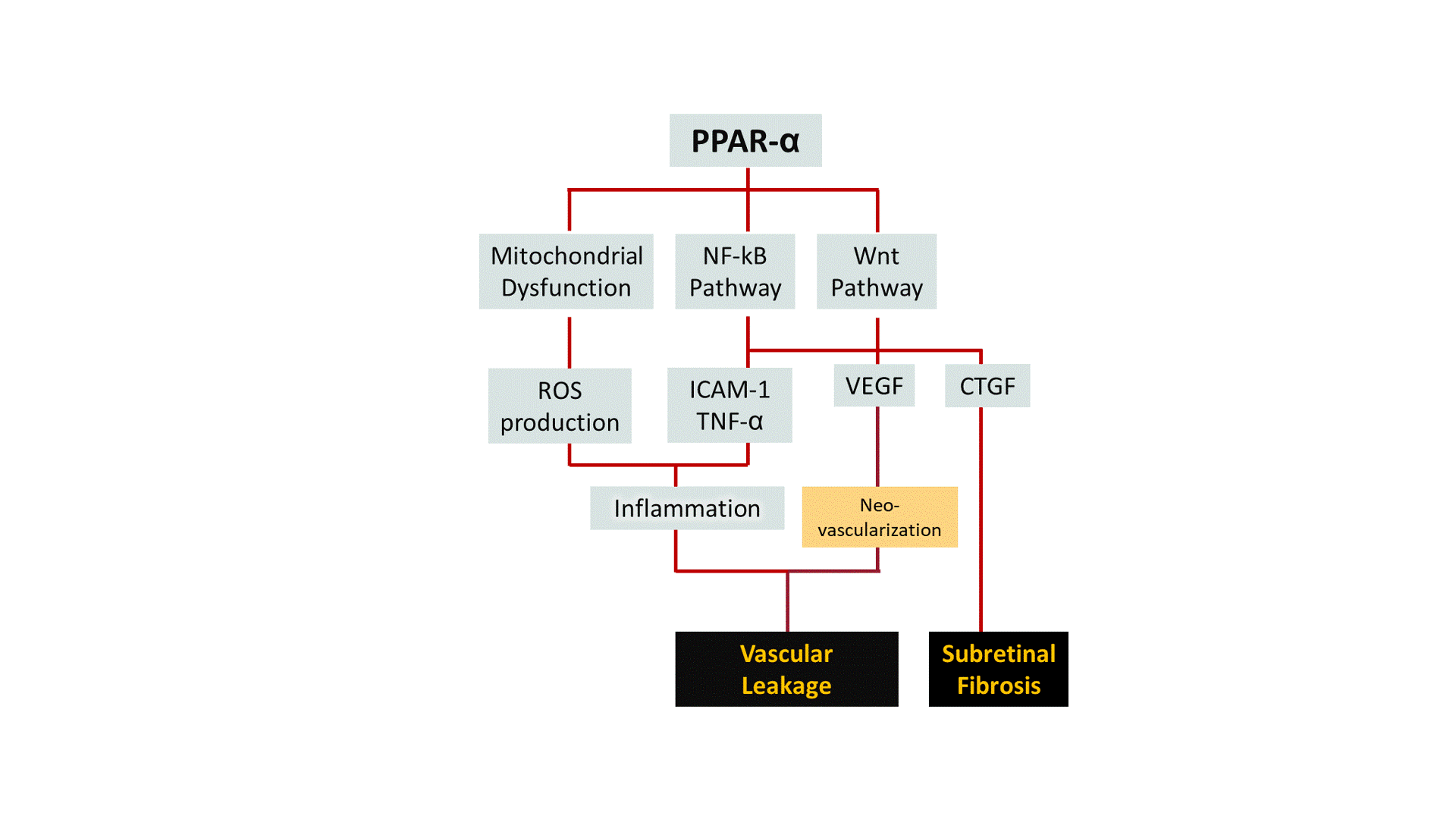
DR is a common cause of vision loss among diabetic patients. Non-proliferative diabetic retinopathy (NPDR) is the early stage of DR during which patients exhibit no obvious symptoms to mild symptoms such as weakened blood vessels. Chronically elevated blood glucose in diabetes can render tiny blood vessels in the eye "leaky", causing fluid to build up in light-sensing tissue called the retina. This phenomenon, known as edema, can greatly affect the central vision when occurs at the region of the retina known as the macula, which is responsible for sharp, color vision. Diabetic Macular Edema (DME) is the most common cause of vision loss in the DR population. The more advanced stage of DR, known as proliferative diabetic retinopathy (PDR), is characterized by abnormal blood vessel growth (i.e. neovascularization). Abnormal vessel function and growth may contribute to retinal inflammation, ischemia (i.e. inadequate blood flow), and neuronal cell death, all of which can ultimately lead to substantial vision loss or complete blindness.

Unmet needs for DR/DME
Although few treatment options exist for DR, current standard of care suffers from critical challenges: 1) nearly 40% of DME patients remain unresponsive to anti-VEGF therapy (i.e., the golden standard) even after 6 months of treatment; 2) current anti-VEGF treatments are cost-prohibitive for many patients (~$1000/shot) and require repetitive intravitreal injections ever 4-8 weeks; 3) secondary approaches like laser photocoagulation create irreversible damage to the retina; and 4) complementary corticosteroid therapy can trigger local/systemic side effects. Hence, we believe finding a new therapeutic target and new therapeutic agents are critical to improving current standard of care for DR.
Developing oral medicine for DR/DME
A growing body of evidence points out that pharmacological activation of Peroxisome Proliferator-Activated Receptor-α (PPAR-α) ameliorates symptoms of DR in both pre-clinical and clinical studies. We are developing structurally unique small-molecule PPAR-α agonists with improved potency, target specificity, efficacy, and safety. By working outside and upstream of VEGF, we aim to broaden the disease modification profile for DR/DME with PPAR-α pharmacotherapy.
Pipeline

bottom of page